Word Equation Of Decomposition Reaction
The reactions involved are. However the decomposition takes place very slowly.
 Chemical Reactions 4 Of 11 Decomposition Reactions An Explanation Youtube
Chemical Reactions 4 Of 11 Decomposition Reactions An Explanation Youtube
Here is one more category of decomposition reactions.

Word equation of decomposition reaction. The general form for a decomposition reaction is. C12H22O11 s H2SO4 aq 12 O2 g 11 C s CO2 g 12 H2O g SO. The general equation for the reaction is AB A B.
H 2O CO 2 Another decomposition reaction occurs when water H 2O breaks down to produce hydrogen H. The equation for this reaction is. This website uses cookies to ensure you get the best experience.
Reactant XY reactant X reactant Y. The opposite of this type of reaction is a synthesis in which simpler reactants combine to form a more complex product. Examples of decomposition reactions include the breakdown of hydrogen peroxide to water and oxygen and.
Equation for decomposition of sugar is. When potassium chlorate is heated in the presence of manganese dioxide as a catalyst it decomposes to give potassium chloride and oxygen. For this reaction we have a decomposition reaction.
Ammonium nitrate Nitrogen gas Oxygen gas Water. Youll need to be patient balancing this equation. In this decomposition reaction NH4NO3 is breaking apart decomposing into N2 O2 H2O.
Some common examples of decomposition reactions are provided below. AB - A B. AB A B.
This is an experiment most students in chemistry lab are familiar with. Where AB is the parent molecule reactant and A B are the product molecules. A decomposition reaction occurs when one reactant breaks down into two or more products.
TERMS IN THIS SET 8 Decomposition reaction definition. Warm²Up 9Translate the word equation into a chemical equation³ identify the type of reaction³ and balance the chemical equation. AB A B Decomposition reactions are also known as analysis reactions or chemical breakdowns.
Equations Inequalities Simultaneous Equations System of Inequalities Polynomials Rationales Coordinate Geometry Complex Numbers PolarCartesian Functions Arithmetic Comp. Decomposition can be achieved by 1. Thermal decomposition of Potassium Chlorate.
HX MHCO 3 MX CO 2 H 2 O. This occurs when you open a can of soft drink and some of the carbon dioxide fizzes out. Which of the following word equations describes the chemical reaction that has occurred in.
Decomposition reactions occur when a single compound breaks apart into two or more substances. This can be represented by the general equation. Decomposition of baking soda word equation Sodium bicarbonate sodium carbonate water hydrogen carbonate.
A reaction in which a compound is seperated into smaller chemical species. The resulting products are water and oxygen gas. In this reaction a single substance.
Hydrogen peroxide decomposes easily and the most stable products are water and oxygen. Free Chemical Reactions calculator - Calculate chemical reactions step-by-step. Type of Chemical Reaction.
In a decomposition reaction we have a single reactant giving at least two products. Asked Aug 8 2019 in Class X Science by aditya23 -2138 points. Using Word Equations to Describe the Decomposition of Hydrogen Peroxide H2O2 Chemistry An experiment shows that hydrogen peroxide decomposes to form water and oxygen gas.
The balanced equation of the decomposition reaction of hydrogen peroxide is that 2H2O2 decomposes into the products 2H2O O2 g. A decomposition reaction takes place when carbonic acid breaks down to produce water H 2O and carbon dioxide CO 2. The general format of a decomposition reaction is provided below.
Decomposition reactions happen all around us but we often dont notice them. Substance AB decomposes or breaks apart producing substances A and B. Word Equation Example hydrochloric acid sodium hydrogencarbonate sodium bicarbonate sodium chloride carbon dioxide water.
Write one equation each for the decomposition reactions where energy is supplied in the form of a heat b light and c electricity. Chemical Equation Example HCl NaHCO 3 NaCl CO 2 H 2 O. Acid hydrogencarbonate bicarbonate salt carbon dioxide water.
Ca OH 2 --- CaO H 2 O NaOH --- Na 2 O H 2 O HNO 3 --- N 2 O 5 H 2 O. In a decomposition reaction a chemical substance is broken down into simpler substances.
 What Is The Chemical Equation For The Decomposition Of Sulfuric Acid Quora
What Is The Chemical Equation For The Decomposition Of Sulfuric Acid Quora
 Combination And Decomposition Reaction Video Khan Academy
Combination And Decomposition Reaction Video Khan Academy
 Balancing Chemical Equations Decomposition Reactions Youtube
Balancing Chemical Equations Decomposition Reactions Youtube
Write Minimum Two Equations Each For Decomposition Reactions Where Energy Is Supplied In The Form Of Heat Light And Electricity Studyrankersonline
 Chemical Reactions Chapter Ppt Video Online Download
Chemical Reactions Chapter Ppt Video Online Download
Decomposition Reaction Types And Classification Of Decomposition Reaction
 Ob All About Decomposition Reactions Decomposition Reactions Are The Opposite Of Synthesis Where A Larger Reactant Is Broken Down Into 2 Or More Smaller Ppt Download
Ob All About Decomposition Reactions Decomposition Reactions Are The Opposite Of Synthesis Where A Larger Reactant Is Broken Down Into 2 Or More Smaller Ppt Download
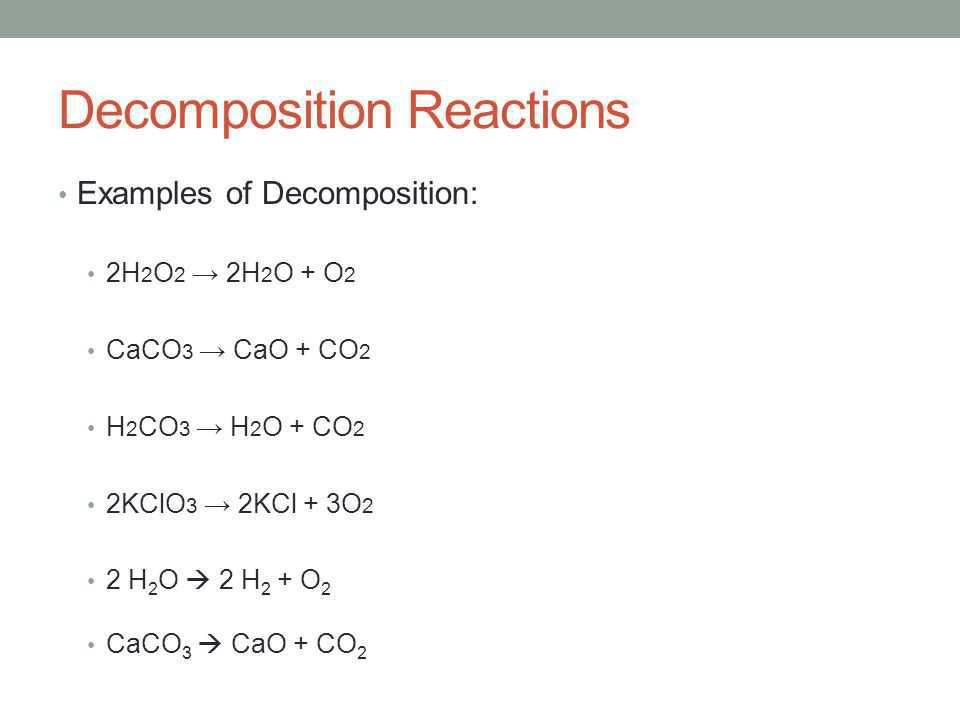 Chemical Reactions And Equations For Class 10
Chemical Reactions And Equations For Class 10
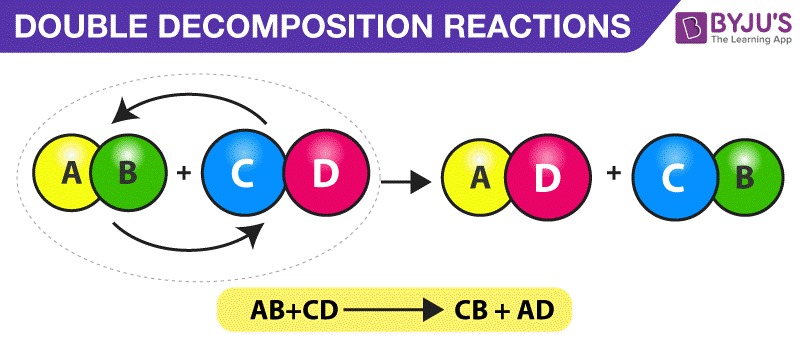 Decomposition Reaction Definition Types Examples Uses
Decomposition Reaction Definition Types Examples Uses
 Chemical Formulae Types Of Reactions Chemical Equations And Balancing Ppt Download
Chemical Formulae Types Of Reactions Chemical Equations And Balancing Ppt Download
 Type Of Reaction For Mgco3 Mgo Co2 Youtube
Type Of Reaction For Mgco3 Mgo Co2 Youtube
 Chemical Equations Classifying Predicting Balancing Ppt Download
Chemical Equations Classifying Predicting Balancing Ppt Download
 Chemical Reactions Anatomy And Physiology I
Chemical Reactions Anatomy And Physiology I
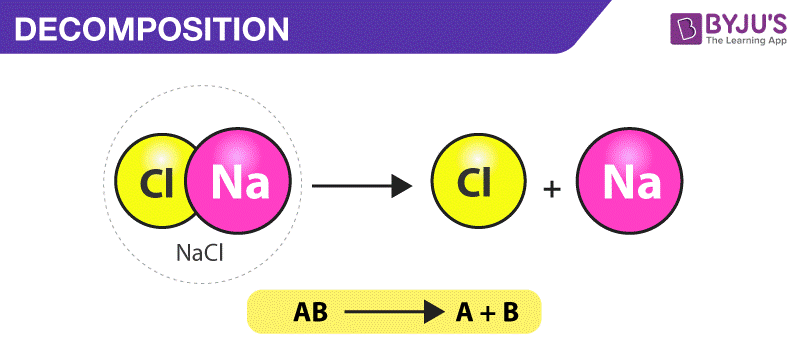 Decomposition Reaction Definition Types Examples Uses
Decomposition Reaction Definition Types Examples Uses
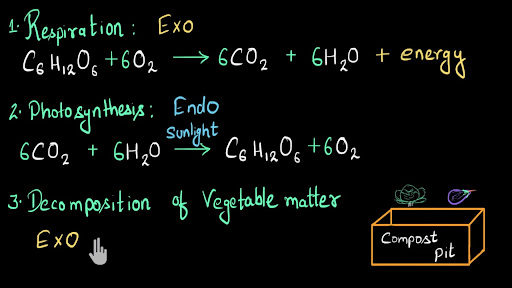
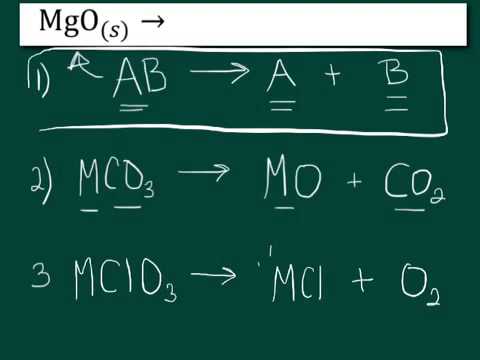 Predicting The Products Of A Chemical Reaction Decomposition Reaction Youtube
Predicting The Products Of A Chemical Reaction Decomposition Reaction Youtube
 Types Of Chemical Reactions P Ppt Download
Types Of Chemical Reactions P Ppt Download
5 Examples Of Decomposition Reaction
Post a Comment for "Word Equation Of Decomposition Reaction"